When you pick up a prescription, you might not realize that the pill in your hand could be a copy of a brand-name drug costing a fraction of the price. That’s a generic drug-and how countries manage them tells a powerful story about healthcare, money, and fairness. Around the world, governments are wrestling with the same problem: how to make life-saving medicines affordable without killing innovation or risking patient safety. The answers vary wildly-from strict price controls in China to market-driven competition in the U.S.-and the outcomes are anything but uniform.
Why Generics Matter More Than Ever
By 2025, the global market for generic drugs hit $468 billion. That’s not just a number-it’s millions of people who can afford insulin, blood pressure meds, or antidepressants because generics exist. The World Health Organization says smart generic policies can slash pharmaceutical spending by 30% to 80%. In the U.S., Medicare saved $142 billion in 2025 thanks to generics-$2,643 per beneficiary. In Europe, generics make up 65% of all prescriptions but only 22% of total drug spending. That’s the power of competition after patents expire.
But here’s the catch: not all generics are created equal. Some countries have rock-solid systems. Others are struggling with shortages, quality issues, or confusing rules. The difference isn’t luck-it’s policy design.
The U.S. Model: High Volume, Low Prices, But Still High Costs
The United States fills 90.1% of prescriptions with generics-the highest rate among developed nations. The FDA has approved over 11,300 generic products as of late 2024. The system works because of the Abbreviated New Drug Application (ANDA) pathway, which lets manufacturers skip costly clinical trials if they prove their drug is bioequivalent to the brand version.
But here’s the paradox: even with near-universal generic use, the U.S. still pays more for drugs than any other country. Why? Because the brand-name drugs-the ones generics replace-are priced sky-high. The net price for public-sector prescriptions is 18% lower than in peer nations, but that’s only because generics drag the average down. The real cost comes from new, patented medicines that insurers and Medicare are forced to pay for.
Still, the U.S. has tools that work. The Competitive Generic Therapy (CGT) designation speeds up approval for drugs with little or no competition. Zenara Pharma’s generic version of Sertraline, approved in August 2025, got its CGT status and hit the market in under a year. That’s faster than most countries can manage.
Europe: Harmonized Rules, Fragmented Prices
The European Union has one of the most sophisticated regulatory systems. The European Medicines Agency (EMA) approves generics for all 27 member states. Sounds efficient, right? Not quite.
Each country sets its own prices. That means the exact same generic pill can cost 300% more in one country than in its neighbor. Germany uses mandatory substitution-pharmacists must swap in generics unless the doctor says no. Italy? Only 67% of prescriptions are filled with generics. The difference isn’t patient preference. It’s policy.
Some countries game the system. The Netherlands uses external reference pricing, picking non-EU countries like Norway and the UK as price benchmarks. By choosing countries with lower prices, Dutch authorities force manufacturers to undercut even their own European competitors. It’s clever, but it creates instability. Manufacturers can’t plan for long-term profits.
China’s VBP: The Nuclear Option
China’s Volume-Based Procurement (VBP) policy is the most aggressive cost-control system on Earth. Starting in 2018, the government stopped letting hospitals choose which generics to buy. Instead, it held centralized auctions. Manufacturers bid to supply 80% of the country’s hospital demand for a specific drug-and the lowest bidder wins.
Results? Average price cuts of 54.7%. In some cases, like certain cancer drugs, prices dropped by 93%. By 2025, over 400 drugs had been through VBP. The catch? Many manufacturers are losing money. A 2025 survey found 23% of generic makers operating at a negative margin on VBP contracts. Some stopped production entirely.
That led to shortages. In 2024, Amlodipine-a common blood pressure drug-was unavailable in 12 provinces for weeks. Patients panicked. The government had prioritized price over supply chain resilience. It’s a lesson in what happens when you squeeze margins too hard.

India: The Pharmacy of the World
India produces 20% of the world’s generic drugs by volume. It’s the go-to source for low-cost medicines in Africa, Latin America, and Southeast Asia. That’s thanks to its 1970 Patents Act, which lets local companies copy drugs as soon as the patent expires-even if the original maker objects.
But there’s a dark side. Between 2022 and 2024, the U.S. FDA issued 17% more warning letters to Indian generic manufacturers over data integrity issues. Some labs were falsifying bioequivalence tests. The Access to Medicine Foundation warns that chasing low prices without strong oversight risks global trust in Indian-made drugs.
Still, for millions who can’t afford branded meds, India is a lifeline. The challenge? Balancing volume with quality. The Central Drugs Standard Control Organization (CDSCO) has cut approval times from 36 months in 2019 to 14 months in 2025. That’s progress-but enforcement still lags.
South Korea: The Middle Ground
South Korea tried something different. In 2020, it launched the “1+3 Bioequivalence Policy.” Only the first generic to enter the market gets full approval. After that, only three more can be approved using the same data. No more copycats flooding the market.
It worked. Between 2020 and 2024, redundant generic entries dropped by 41%. But it also hurt competition. New generic launches fell by 29%. The government responded with a pricing tier system: generics that meet both quality and price standards get 53.55% of the brand price. Those meeting only one criterion get 45.52%. The rest? Just 38.69%.
It’s a smart compromise. It limits market chaos while still pushing prices down. But it’s also a warning: too much control can stifle innovation. Fewer companies are willing to invest in generics if they know they’ll be locked out after the first few entries.
Japan: The Slow Burn
Japan doesn’t have a flashy policy. It just cuts prices-every two years-for both branded and generic drugs. The result? Generic use is high (76.8% by volume), but the market barely grows. Manufacturers can’t raise prices, so they don’t invest in new generics. Innovation stalls. It’s a system designed to keep costs flat, not to grow access.
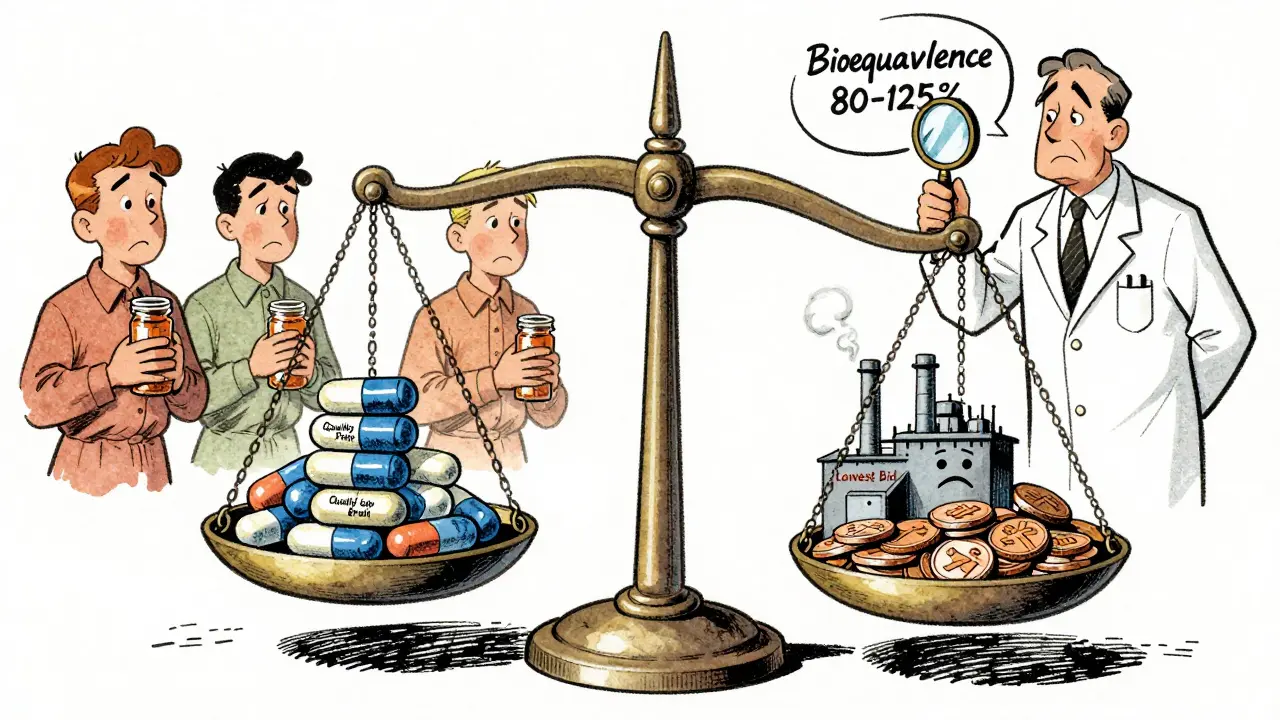
What Works? The Common Threads
Across all successful systems, three things stand out:
- Clear bioequivalence standards: All generics must prove they deliver the same amount of drug into the bloodstream as the brand, within 80-125% of the original. No exceptions.
- Doctor and pharmacist education: When providers understand generics are safe, patient acceptance jumps by 22-35%. Fear of inferior quality is the biggest barrier-not science.
- Reasonable margins: If manufacturers can’t make at least 15-20% gross profit, they cut corners or quit. The WHO says sustainable pricing isn’t about the lowest bid-it’s about keeping factories running.
The Future: More Pressure, More Risk
Between 2025 and 2030, over $200 billion in branded drug sales will lose patent protection. That’s a gold rush for generics-if the system allows it.
The U.S. Inflation Reduction Act is starting to change the game. By 2028, Medicare will negotiate prices for 10-20 high-cost drugs each year. That could push even more patients toward generics.
Meanwhile, the EU is pushing a new Pharmaceutical Package to harmonize pricing rules. If it passes, we might finally see fewer price gaps between neighboring countries.
But the biggest threat isn’t policy-it’s quality erosion. FDA import alerts for bad generic manufacturing jumped from 1,247 in 2020 to 2,183 in 2024. As margins shrink, some factories cut testing, skip inspections, or falsify data. That’s not just a business risk. It’s a public health risk.
What Patients Should Know
If you’re taking a generic drug, you’re likely saving hundreds or thousands a year. Most generics work just as well as the brand. But if you’re on a narrow therapeutic index drug-like warfarin, levothyroxine, or epilepsy meds-talk to your doctor. Small differences in absorption can matter.
And don’t assume your insurance always favors generics. In the U.S., some Pharmacy Benefit Managers (PBMs) charge higher copays for generics than for brand-name drugs. It’s a loophole. Check your formulary.
Patients in Europe and Asia report high satisfaction-when pharmacists explain the switch. Communication matters. A simple note: “This is the same medicine, cheaper. It’s safe.” makes all the difference.
Are generic drugs really as effective as brand-name drugs?
Yes. By law, generics must deliver the same active ingredient, in the same strength, and at the same rate as the brand-name version. The FDA and EMA require bioequivalence testing-meaning the drug enters your bloodstream at nearly identical levels. Studies show generics perform the same in real-world use. The only exceptions are rare cases with narrow therapeutic index drugs, where even small variations matter. Always consult your doctor if you’re switching for the first time.
Why do some countries have cheaper generics than others?
It’s all about policy. Countries like China use bulk buying to force prices down. The Netherlands picks low-price countries as benchmarks. The U.S. lets market competition drive prices, but only after patents expire. In places without strong price controls, manufacturers can charge more. It’s not about manufacturing cost-it’s about who’s setting the rules.
Can generic drugs cause more side effects?
No, not because they’re generic. The active ingredient is identical. But inactive ingredients-like fillers or dyes-can vary. For most people, this doesn’t matter. But if you have allergies or sensitivities, you might react to a different filler. If you notice new side effects after switching, talk to your pharmacist. It’s not the drug’s effectiveness-it’s the formulation.
Why are there shortages of generic drugs?
Shortages happen when manufacturers can’t make money. In places like China, VBP drives prices below production cost. In India, quality crackdowns shut down factories. In the U.S., low profit margins mean some companies stop making older generics because it’s not worth the effort. It’s not a supply chain issue-it’s an economic one. When margins disappear, production stops.
Will generic drugs become even cheaper in the future?
Some will, some won’t. Countries with aggressive price controls will keep pushing down prices-but that risks quality and supply. Meanwhile, new generics for complex drugs (like biologics) will be more expensive to make. The real trend isn’t lower prices across the board-it’s more competition for simple drugs and higher prices for complex ones. The future belongs to manufacturers who can make high-quality generics profitably, not just cheaply.
What Comes Next?
If you’re a patient, stick with generics when you can. They’re safe, effective, and save you money. If you’re a policymaker, remember: the goal isn’t the lowest price-it’s sustainable access. A factory that shuts down because it can’t make a profit doesn’t help anyone.
The next decade will test global systems. Will countries protect quality while cutting costs? Will innovation keep up as patents expire? The answer will shape whether millions can afford their medicines-or whether they’re forced to choose between pills and rent.





16 Comments
Generics are just brand-name drugs with different fillers. FDA bioequivalence is a joke. 80-125% range? That’s not medicine, it’s gambling.
Oh wow, the U.S. pays the most for drugs but uses the most generics? That’s like buying the cheapest sneakers in the world… while wearing a $5,000 suit. 🤡
Let’s be real: the entire generic system is a neoliberal farce. Capitalism doesn’t care if you live or die-it cares about margin compression and shareholder dividends. The fact that we’re celebrating 54% price cuts as ‘success’ is like patting a starving dog for not biting you yet.
China’s VBP isn’t bold-it’s brutal. And India’s FDA warning letters? That’s not a ‘quality issue,’ it’s the inevitable collapse of a profit-driven global pharma supply chain. We’re outsourcing our health to sweatshops with lab coats.
The ‘1+3’ policy in Korea? Genius. But only because it acknowledges that infinite competition = infinite decay. When you commodify life-saving medicine, you don’t get innovation-you get corner-cutting, black-market fillers, and patients dying because their amlodipine was made in a basement with a 3D printer.
And don’t get me started on PBMs. They’re the real villains. Charging more for generics? That’s not a loophole-it’s a felony. The entire system is a Rube Goldberg machine designed to extract wealth from the sick.
Meanwhile, the WHO says ‘reasonable margins’? Cute. What’s reasonable? 15%? Try 200% for the brand-name oligopolists. The hypocrisy is nauseating.
Patients need to stop being grateful for crumbs. This isn’t about ‘affordability.’ It’s about dignity. And dignity doesn’t come from a 76% discount on a pill that should’ve cost $0.02 to produce.
India’s 1970 Patents Act was a revolutionary act of epistemic justice. The West built its wealth on stolen knowledge-now they cry foul when the Global South dares to replicate what they patented for profit. This isn’t piracy-it’s reparations.
Yes, there are quality issues. But let’s not pretend the FDA’s own labs aren’t underfunded, or that U.S. manufacturers don’t outsource to the same factories they now condemn. The moral panic is performative. The real crime? Letting corporations own the blueprint of human survival.
Generics aren’t ‘cheaper drugs.’ They’re the only thing standing between poverty and death for 3 billion people. If you’re not screaming about this, you’re complicit.
Biggest thing people miss? It’s not about the drug-it’s about the pharmacist. When my grandma switched to generic levothyroxine, she got dizzy. Not because it was bad-because no one told her to stick with the same brand. A simple ‘this is the same, just cheaper’ note changed everything. Communication > regulation.
Also, if your insurance charges more for generics? Switch plans. PBMs are scammers. I literally printed a comparison chart and mailed it to my rep. They changed it next month. 🤷♂️
China’s VBP is terrifying… and beautiful. Imagine a world where medicine isn’t a luxury. Where a grandmother in rural Sichuan gets her blood pressure pill for 2 cents. That’s not policy-it’s poetry.
Yes, factories fold. Yes, shortages happen. But ask yourself: would you rather have 100% access at 50% cost… or 80% access at 100% cost? The math is clear. The moral is harder.
India feeds the world. The U.S. patents it. Europe haggles. Japan naps. And we’re still surprised when someone dies because they couldn’t afford a pill?
Let’s stop calling this ‘healthcare.’ It’s a market. And markets don’t care about lives. Only margins.
Why are we even talking about this? It’s obvious. The U.S. is a banana republic with a healthcare system. You pay more for insulin than a Tesla. And you call this freedom? 😂
Meanwhile, in South Africa, we get Indian generics for $1 a month. No drama. No PBMs. Just medicine. Maybe we should stop looking to America for solutions. 🤷♀️
What’s fascinating is the hidden variable: bioequivalence thresholds. The 80-125% range was established in the 1970s based on pharmacokinetic models from a sample size of 24 healthy males. We’ve had 50 years of pharmacogenomics, AI-driven absorption modeling, and real-world evidence-but we still use the same arbitrary window.
That’s not science. That’s bureaucratic inertia. And it’s why some patients report ‘different effects’-not because generics are inferior, but because the standard is archaic.
We need dynamic bioequivalence: adaptive thresholds based on drug class, patient phenotype, and therapeutic index. Otherwise, we’re treating a population of 8 billion with a 1973 algorithm.
Europe’s fragmented pricing is a mess… but honestly? It’s kind of beautiful. Germany forces substitution-brilliant. Italy doesn’t-also fine. The Netherlands benchmarking Norway? Genius. It’s not chaos-it’s diversity. Let 27 nations experiment. See what works. Then scale it.
Also, I’m just here to say: thank you, India. Seriously. My dad’s COPD meds? Made in Hyderabad. Cost $12 a month. In the U.S.? $800. I don’t care about ‘intellectual property’-I care about my dad breathing. 💙
The WHO’s 15-20% margin recommendation is dangerously naive. In India, a generic manufacturer’s gross margin on amlodipine is 8%. They survive because they make 200 other drugs. Profit isn’t the goal-it’s survival. And in a country where 70% of healthcare is out-of-pocket, even 8% is a luxury.
Policy must account for ecosystem economics, not just single-drug margins. A factory that runs at 5% profit while supplying 50 million pills is more valuable than one running at 25% profit while making 500,000.
Also, the ‘quality erosion’ narrative is weaponized. The FDA’s import alerts rose because they’re inspecting more-not because quality dropped. China and India are improving. The U.S. is just scared of competition.
Stop framing this as ‘good vs bad’ systems. It’s about scale, equity, and political will. And until we treat medicine as a public good-not a commodity-we’re all losing.
Let’s not romanticize India. Yes, they make 20% of the world’s generics-but their regulatory system is still catching up. I’ve seen labs where the same sample is tested three times until they get the ‘right’ result. That’s not innovation. That’s fraud.
And China’s VBP? It’s like a dictatorship of cost-cutting. No wonder they’re having shortages. You can’t run a factory on negative margins. People aren’t machines.
The real solution? A global generics consortium. Joint R&D. Shared quality control. Profit-sharing models. Not auction wars. Not patent theft. Collaboration.
We need a new paradigm. Not ‘lowest price wins.’ But ‘best value for humanity.’
My cousin in rural Kentucky got her diabetes meds switched to generic metformin. She called me crying because her blood sugar spiked. Turned out the new version had a different filler-she’s allergic to corn starch. No one warned her.
Generics aren’t magic. They’re medicine. And medicine needs context. Education. Communication. Not just price tags.
I live in a country where generics are 90% of prescriptions. We don’t have panic. We don’t have shortages. Why? Because doctors trust them. Pharmacists explain them. Patients feel safe.
The real difference isn’t policy-it’s culture. We don’t treat medicine like a battlefield. We treat it like a community.
Maybe the answer isn’t more auctions or more patents… but more trust.
Let’s not pretend this is about health. It’s about power. Who controls the molecules that keep us alive? Corporations. Governments. Patent lawyers. The WHO? A nice logo on a brochure.
The U.S. spends $1.3 trillion on healthcare. $468 billion on generics. That’s not a win-it’s a hostage situation. We’re paying for the privilege of being allowed to buy cheap pills while the real profits are locked in patents no one can touch.
And the ‘future’? Biologics. Biosimilars. $100,000 cancer drugs with ‘generic’ versions that cost $80,000. The game is rigged. The players are the same. The victims? Always us.
They say ‘innovation’ is the solution. But innovation without equity is just a new kind of exploitation.
The assertion that generics are ‘as effective’ as brand-name drugs is empirically unsupported. While bioequivalence is statistically demonstrated in controlled trials, real-world pharmacokinetic variability-particularly in polypharmacy patients, the elderly, and those with hepatic impairment-is systematically ignored by regulatory agencies.
Furthermore, the notion that ‘patient education’ resolves adherence issues is a patronizing myth. Cognitive dissonance between perceived efficacy and actual outcomes is well-documented in clinical literature. The placebo effect is not a policy tool.
It is not ‘fairness’ to mandate substitution when the therapeutic index is narrow. It is negligence.
And to those who invoke ‘India as a lifeline’-please cite the mortality studies. The 2023 Lancet paper on Indian-manufactured antiretrovirals showed a 12% higher failure rate in sub-Saharan Africa compared to EU-sourced equivalents. This is not humanitarianism. It is risk externalization.
And yet, in the same breath, the West refuses to license these same drugs for domestic production. They call India’s system ‘unethical’-but refuse to let their own citizens make them. Hypocrisy isn’t a flaw. It’s the business model.
Write a comment