Biosimilar Interchangeability Checker
Is this biosimilar interchangeable?
Check if a biologic drug has an FDA-designated interchangeable biosimilar and understand what this means for pharmacy substitution.
How it works
This tool uses the FDA Purple Book data to show which products have an interchangeable designation. Note that state pharmacy laws may still require prescriber notification or approval even if FDA approves interchangeability.
As of 2025, only 7 interchangeable biosimilars are approved by the FDA across 3 therapeutic areas:
- 2 insulin products
- 3 treatments for inflammatory diseases
- 2 therapies for eye conditions
The Purple Book isn’t a novel you read on the couch-it’s the U.S. Food and Drug Administration’s official, searchable database that tells you exactly which biological drugs are approved, which ones are biosimilars, and which ones can be swapped at the pharmacy like generics. If you’re a pharmacist, a prescriber, or even a patient trying to understand why your insulin prescription changed, this is the resource that makes sense of it all.
What Exactly Is the Purple Book?
The Purple Book is the FDA’s public record of all licensed biological products in the United States. It includes the original brand-name biologics, their biosimilar versions, and the rare subset called interchangeable biosimilars. Before 2020, this information was split across two separate lists-one for drugs regulated by CDER (like antibodies and hormones) and another for CBER products (like vaccines and cell therapies). Now, it’s all in one place, updated in real time, and searchable by product name, manufacturer, or status. It’s not just a list. Each entry has key details: the date the product was approved under section 351(a) of the Public Health Service Act, whether it’s protected by exclusivity, and crucially-whether it’s been designated as biosimilar or interchangeable. The database uses color-coded cards: if two products share the same color, they’re linked as reference and biosimilar. You can also see icons showing how the drug is delivered-autoinjector, pre-filled syringe, vial-so pharmacists know what’s physically available.Biosimilar vs. Interchangeable: The Critical Difference
Not all biosimilars are created equal. A biosimilar is a biological product that’s highly similar to an FDA-approved reference product. There might be tiny, harmless differences in inactive ingredients, but no meaningful difference in safety, purity, or potency. Think of it like two different brands of aspirin-same active ingredient, same effect, just different packaging or fillers. An interchangeable biosimilar goes further. To earn that label, the manufacturer must prove that switching back and forth between the biosimilar and the original product won’t increase risk or reduce effectiveness. That means clinical studies where patients were switched multiple times between the two-sometimes every other dose-and still had the same outcomes. The FDA doesn’t say interchangeable biosimilars are better. They just say you can swap them without needing a new prescription each time. This matters because only interchangeable products can be automatically substituted by a pharmacist, just like a generic pill. A regular biosimilar? You still need the doctor to write the brand name or specify "dispense as written."How the FDA Determines Interchangeability
Getting an interchangeable designation isn’t just about proving similarity-it’s about proving switchability. The FDA requires data from clinical studies that show no increased risk when patients alternate between the reference product and the biosimilar. For example, if a patient uses Humira for rheumatoid arthritis and switches to an interchangeable biosimilar, then switches back, their immune response, side effects, and disease control must stay stable. No drop-off. No surprises. These studies are more complex than the ones needed for basic biosimilarity. They often involve multiple switches over months, with careful monitoring. Pfizer’s analysis in 2024 confirmed that this is the key hurdle: most biosimilars pass the similarity test, but only a few have the switch data to earn interchangeability. The FDA is clear: interchangeability doesn’t mean safer or more effective. It just means predictable when switching. And that’s a big deal for patients who might need to change treatments due to cost or availability.State Laws Still Control What Pharmacists Can Do
Here’s where things get messy. Even if the FDA says a biosimilar is interchangeable, your pharmacist might not be allowed to swap it without telling your doctor. That’s because state laws control pharmacy practice, not federal approval. As of 2023, 47 states and Puerto Rico allow pharmacists to substitute an interchangeable biosimilar without contacting the prescriber. But in those states, they still might have to notify the doctor, document the swap, or inform the patient. A few states require prescriber permission every time. Others don’t allow substitution at all unless the prescription says "dispense as written." This creates a patchwork. A patient in Texas might get an interchangeable biosimilar automatically. A patient in California might need a new prescription. A patient in New York might be told the pharmacy can’t substitute at all-even if the FDA says it’s allowed. The FDA’s Purple Book doesn’t track state rules. It only tells you what’s federally approved. So if you’re a pharmacist or a patient, you need to know your state’s laws too.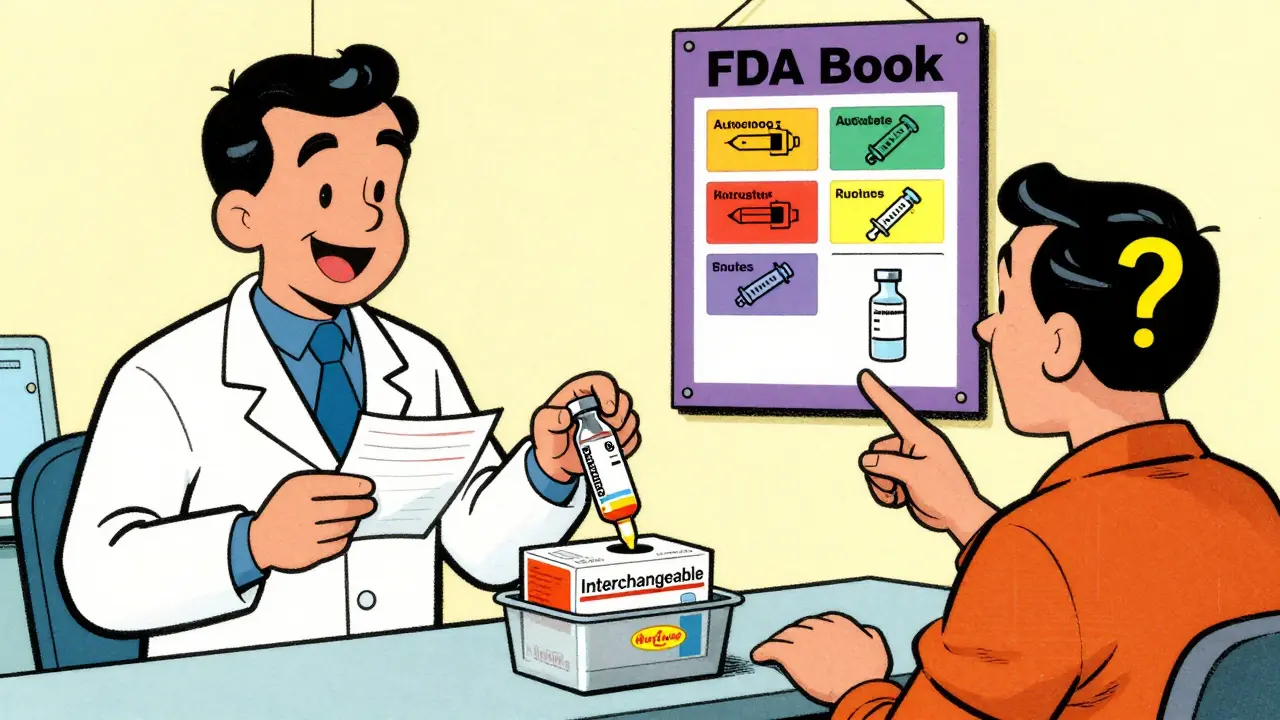
Which Products Are Interchangeable? (As of 2025)
As of late 2023, only seven biosimilars had earned the interchangeable designation from the FDA. By 2025, that number has grown slightly, but it’s still a small fraction of the total biosimilars on the market. Here’s what’s approved so far:- Two insulin products (used for diabetes)
- Three treatments for inflammatory diseases like rheumatoid arthritis and Crohn’s disease
- Two therapies for eye conditions (retinal diseases)
Why the Purple Book Matters for Patients and Providers
For doctors, the Purple Book helps avoid confusion. You can check whether the biosimilar your patient is taking is interchangeable or just biosimilar. That affects how you write prescriptions and whether you need to follow up after a switch. For pharmacists, it’s a daily tool. When a prescription comes in for a biologic, they pull up the Purple Book to see if a substitution is legally allowed. If the product is interchangeable, and state law permits it, they can swap it without calling the doctor. For patients, it’s about transparency. If you’re paying out of pocket for a $20,000 biologic, knowing there’s a cheaper, FDA-approved interchangeable version could save you thousands. But you won’t know unless you-or your provider-look it up. The FDA designed this tool to cut through the noise. No marketing hype. No vague claims. Just facts. And that’s rare in drug pricing.What’s Next for Biosimilars?
More applications for interchangeability are coming. Companies are investing in the complex switching studies because the payoff is bigger: automatic substitution means faster market uptake. Insurers are starting to favor interchangeable biosimilars in formularies because they’re cheaper and legally substitutable. The FDA is also updating guidance on labeling to make sure biosimilar packaging clearly states whether it’s interchangeable. No more confusion on the shelf. But adoption still lags. Many prescribers don’t know how to use the Purple Book. Many patients don’t know their insulin can be swapped. And many pharmacies still default to the brand name because they’re unsure of the rules. The Purple Book is the foundation. But real change happens when patients ask for it, when pharmacists use it, and when doctors trust it.
How to Use the Purple Book
It’s free. It’s online. And it’s easy:- Go to fda.gov/purplebook (no login needed).
- Search by brand name (e.g., "Enbrel") or active ingredient.
- Look for the color-coded cards. Matching colors = biosimilar or interchangeable.
- Check the "Product Status" column. It will say "351(k) Interchangeable" if it’s substitutable.
- Click on a product card to see details: manufacturer, date licensed, exclusivity status, delivery method.
Common Misconceptions
- "Interchangeable means better." No. It means switchable. Safety and effectiveness are the same as the reference product.
- "All biosimilars can be swapped." Only the ones labeled "interchangeable" by the FDA-and only if your state allows it.
- "The Purple Book is for doctors only." Anyone can use it. Patients, pharmacists, even students.
- "Unbranded biologics are interchangeable." No. The FDA doesn’t use that term. If it’s not labeled "interchangeable," it’s not automatically substitutable.
Final Thoughts
The Purple Book is one of the most underused tools in modern medicine. It’s not flashy. It doesn’t have ads. It doesn’t try to sell you anything. It just tells you what’s approved, what’s interchangeable, and who made it. For a system that spends billions on biologics every year, this level of transparency is rare. And it’s exactly what’s needed to bring down costs without compromising safety. If you’re in healthcare, bookmark it. If you’re a patient, ask your pharmacist if your biologic has an interchangeable version. And if you’re a prescriber-start using it when you write prescriptions. The future of affordable biologics isn’t in marketing. It’s in this database.What is the Purple Book used for?
The Purple Book is the FDA’s official database that lists all licensed biological products in the U.S., including reference products, biosimilars, and interchangeable biosimilars. It helps healthcare providers, pharmacists, and patients identify which products are approved as biosimilar or interchangeable, and whether substitution is permitted under federal guidelines.
Can any biosimilar be swapped for the original drug at the pharmacy?
No. Only biosimilars that have received an official "interchangeable" designation from the FDA can be substituted without a new prescription. Even then, state pharmacy laws control whether substitution actually happens. Some states require pharmacist notification or prescriber consent.
How is an interchangeable biosimilar different from a regular biosimilar?
A regular biosimilar is highly similar to the reference product with no clinically meaningful differences in safety or effectiveness. An interchangeable biosimilar must prove, through additional clinical studies, that switching back and forth between it and the original product doesn’t increase risk or reduce effectiveness. This allows pharmacists to substitute it automatically under state law.
Are interchangeable biosimilars safer or more effective than regular biosimilars?
No. The FDA states clearly that interchangeability does not mean the product is safer or more effective. It only means it can be substituted without additional risk when switched multiple times. Both types meet the same high standard for similarity to the reference product.
Where can I find the Purple Book and is it free to use?
The Purple Book is free and publicly accessible at fda.gov/purplebook. It’s searchable by product name, manufacturer, or status. No registration is required. It’s updated weekly and includes color-coded product cards, delivery methods, and interchangeability status.
Why aren’t more biosimilars interchangeable?
Because proving interchangeability requires additional clinical studies-specifically, switching studies that show repeated alternation between the biosimilar and the reference product doesn’t affect safety or effectiveness. These studies are expensive and complex, so many manufacturers focus only on basic biosimilarity to enter the market faster.
Do state laws override the FDA’s interchangeability designation?
Yes. The FDA grants the interchangeable designation at the federal level, but state pharmacy laws determine whether pharmacists can actually substitute the product. Some states allow automatic substitution, others require prescriber approval or patient notification. Pharmacists must follow their state’s rules, even if the FDA says a product is interchangeable.





13 Comments
So let me get this straight - we’re paying $20K for insulin because Big Pharma doesn’t want us to know we can swap it for half the price? And the FDA’s got this shiny purple book but pharmacies still act like it’s a secret cult manual? I’m not mad, I’m just disappointed.
Also, why is it called the Purple Book? Did someone at the FDA have a meltdown in a crayon box?
Also also - if I’m a patient and my pharmacist swaps my Humira without telling me, am I legally allowed to scream into the void and demand a refund in glitter?
Let’s be clear: interchangeable ≠ better. It means the FDA finally got tired of pharmaceutical companies playing musical chairs with biologics and said, ‘Enough.’ But the real issue? Pharmacists still treat this like it’s a forbidden spell. They’ll swap a generic Advil in a heartbeat, but a biosimilar? ‘Oh no, we need a signed affidavit from your priest.’
Meanwhile, the patients who need this the most? They’re still getting billed for the brand name because nobody bothered to read the damn Purple Book.
It’s not a database. It’s a middle finger to corporate greed - and we’re all too lazy to use it.
Thank you for this clear, essential guide. As someone who has witnessed the financial toll of biologics on loved ones, I am profoundly grateful for the transparency the Purple Book offers. It is not merely a tool - it is an act of justice in a system that often obscures truth behind branding and cost.
Let us all, as patients, providers, and citizens, commit to using this resource. Knowledge is not power - it is dignity.
Who really controls the Purple Book? The FDA says it’s public but have you noticed how the interchangeable biosimilars always seem to come from the same three corporations that also own the original brands?
And why is the database updated weekly but never on weekends? Coincidence? Or is someone at the FDA still on corporate payroll?
Also - why is it purple? Purple is the color of royalty. Are they making us bow to the biologics throne?
They say it’s free. But what’s the hidden cost? Your data? Your trust? Your soul?
Think about it - this isn’t just about insulin or Humira. This is about the entire illusion of choice in American medicine.
We’re told we have options. But the options are all written by the same five lawyers who also wrote the patent extensions.
The Purple Book? It’s a magician’s mirror - it shows you the truth, but only if you’re willing to look past the glitter. And most people? They’d rather believe the hype than face the fact that their life-saving drug was designed to keep them poor.
They call it biosimilar. I call it corporate camouflage.
And the state laws? That’s the real show. The federal government gives you freedom. The states take it away with a signature and a clipboard. Who’s really in charge here? The FDA? Or the lobbyists with the golden pens?
In Nigeria, we don’t even have access to the original biologics, let alone biosimilars. You Americans treat this like a luxury debate - but here, we fight for basic generics.
The Purple Book is a luxury. The fact that you have this database and still don’t use it? That’s your problem, not the system’s.
Stop complaining about substitution rules and start demanding universal access. Until then, your privilege is showing.
I’ve been using the Purple Book for a year now. It’s not perfect, but it’s the only thing keeping me from overpaying for my biologic.
My pharmacist didn’t even know it existed until I showed her. Now she prints out the pages for every patient who asks.
Small wins. But they matter.
Here’s the thing nobody wants to say - the FDA doesn’t care if you can afford your medicine. They care if the data checks out. And the data? It’s all manufactured by the same companies that profit from the original product.
Those switching studies? They’re not designed to test safety - they’re designed to meet regulatory boxes so the company can slap ‘interchangeable’ on the label and raise the price of the original just a little bit higher to compensate.
And don’t get me started on the color-coded cards. Purple. Why not red? Red means danger. Purple means ‘this is fine.’
They’re not giving us transparency. They’re giving us a pretty lie wrapped in a PDF.
And now the states are playing referee? Please. This is a circus where the clowns are writing the rules and the audience is paying for the tickets.
It’s not a database. It’s a placebo for regulatory trust.
And if you think your pharmacist is your ally? They’re just following the script written by a corporate compliance officer who’s never met a patient.
Wake up. This isn’t healthcare. It’s a subscription service with a side of bureaucracy.
It’s funny - we live in a world where you can track your coffee order across three continents but you can’t tell if your insulin is interchangeable without a PhD in regulatory law.
The Purple Book is like the internet’s forgotten library card. It’s there. It’s free. But nobody knows how to use it.
Maybe we need a TikTok tutorial. ‘How to not get ripped off by your pharmacy in 60 seconds.’
Also - purple? Really? Next they’ll make the diabetes app neon pink and call it ‘Empowerment Edition.’
As a regulatory affairs professional, I can confirm that the interchangeability designation requires a level of clinical rigor that exceeds that of basic biosimilarity. The switching studies involve longitudinal monitoring, immunogenicity assessments, and pharmacokinetic equivalence across multiple transitions - often over 6–12 months.
The FDA’s criteria are scientifically sound. The barrier is not bureaucratic inertia - it is the immense cost and complexity of generating such data.
That said, the disparity in state laws remains a critical fragmentation in patient access. Harmonization is needed - not just in labeling, but in practice.
Thank you for this comprehensive and thoughtful overview. As a healthcare provider, I have long advocated for the use of the Purple Book in clinical practice. Its existence is a triumph of transparency in an industry often defined by opacity.
I encourage every clinician to integrate this resource into patient education. Knowledge empowers - and in this context, it may also alleviate financial burden and reduce therapeutic uncertainty.
Let us not underestimate the power of a well-informed patient.
Let’s be real - the Purple Book is just the FDA’s way of saying, ‘We tried.’
It’s like giving someone a map to the treasure and then locking the chest with a 12-digit code only the CEO knows.
And the color-coded cards? So poetic. So symbolic. So utterly useless if your insurance won’t cover the biosimilar unless you fill out Form 7B-Alpha-9 in triplicate.
Meanwhile, the pharmaceutical industry is busy patenting the *color* purple.
They’re not selling drugs anymore.
They’re selling mythology.
And we’re all just clicking ‘Accept’ on the EULA.
So I asked my pharmacist if my Humira could be swapped and she said ‘I don’t know, I’ve never seen that book.’
So I showed her the FDA website.
She said ‘Oh, that’s not real. That’s just for doctors.’
Then she gave me the brand name.
And charged me $2,100.
And I cried.
Not because I’m weak.
Because the system is broken.
And no one cares enough to fix it.
And the Purple Book?
It’s just a pretty lie on a website.
And I’m tired.
Write a comment