Triple-negative breast cancer (TNBC) doesn’t respond to hormone therapy or HER2-targeted drugs. That makes it one of the hardest types of breast cancer to treat. It accounts for about 1 in 10 cases of breast cancer, but it’s responsible for a disproportionate number of deaths. Why? Because it grows fast, comes back often, and until recently, chemotherapy was about the only option. But things are changing-fast.
What Makes TNBC Different?
Most breast cancers have receptors that drugs can latch onto: estrogen, progesterone, or HER2. TNBC has none of them. That means treatments that work for other types just don’t work here. It’s like trying to lock a door with no keyhole. Doctors used to rely on strong chemotherapy to shrink tumors before surgery. But even then, many patients saw the cancer return within three years.
Now, we know TNBC isn’t just one disease. It’s a group of cancers with different genetic fingerprints. Some have BRCA mutations. Others have high levels of PD-L1, a protein that tells the immune system to stand down. A few have unusual gene changes that make them vulnerable to new drugs. That’s why testing isn’t optional anymore-it’s essential.
Standard Treatment Today: Chemo, Then What?
For early-stage TNBC, the standard still starts with chemotherapy. But it’s not the same chemo as 10 years ago. Platinum drugs like carboplatin are now routinely added to regimens, especially for younger patients or those with BRCA mutations. These drugs damage DNA in a way that’s particularly effective against TNBC cells.
After chemo comes surgery-usually a mastectomy or lumpectomy. Then, more chemo or radiation. But here’s the shift: radiation is no longer just a cleanup step. A 2025 study from UT Southwestern showed that giving radiation at the very start of treatment, before most chemo, cuts down the number of immunotherapy doses needed and reduces serious side effects by nearly half.
That’s huge. The old way involved months of chemo, multiple rounds of pembrolizumab (an immunotherapy drug), and then surgery. Many patients ended up with severe fatigue, nerve damage, or low blood counts. The new approach? Two doses of pembrolizumab, radiation, then chemo. Same results. Less toxicity.
Immunotherapy: When It Works and When It Doesn’t
Immunotherapy doesn’t work for everyone. But for those whose tumors express PD-L1 (about 40% of metastatic TNBC cases), it’s a game-changer. Pembrolizumab and atezolizumab are now approved for use with chemotherapy in early and advanced TNBC.
The KEYNOTE-522 trial showed that adding pembrolizumab to chemo before surgery led to a 64.8% pathologic complete response rate in PD-L1-positive patients. That means no live cancer cells were found in the breast or lymph nodes after treatment. For PD-L1-negative patients? Only 44.1%. So testing matters.
But even in PD-L1-positive cases, not all responses last. That’s why researchers are testing combinations-like pairing immunotherapy with drugs that target other immune checkpoints, or with vaccines that train the body to recognize cancer-specific mutations.
PARP Inhibitors: Targeting BRCA Mutations
About 1 in 5 TNBC patients carry a BRCA1 or BRCA2 gene mutation. These genes help fix damaged DNA. When they’re broken, cancer cells struggle to repair themselves. PARP inhibitors like olaparib and talazoparib exploit that weakness.
In the OlympiAD trial, women with BRCA mutations who took olaparib lived 7.8 months longer without their cancer growing compared to those on standard chemo. Side effects? Anemia, nausea, fatigue. Manageable. But here’s the catch: only patients with inherited (germline) BRCA mutations benefit. Tumor-only testing won’t cut it. You need a blood or saliva test.
Even more promising? Combining PARP inhibitors with other drugs. Early studies show that blocking CDK12 or CDK6 alongside PARP inhibition can double tumor shrinkage in lab models. These are still in trials, but they hint at a future where we hit cancer with multiple targets at once.
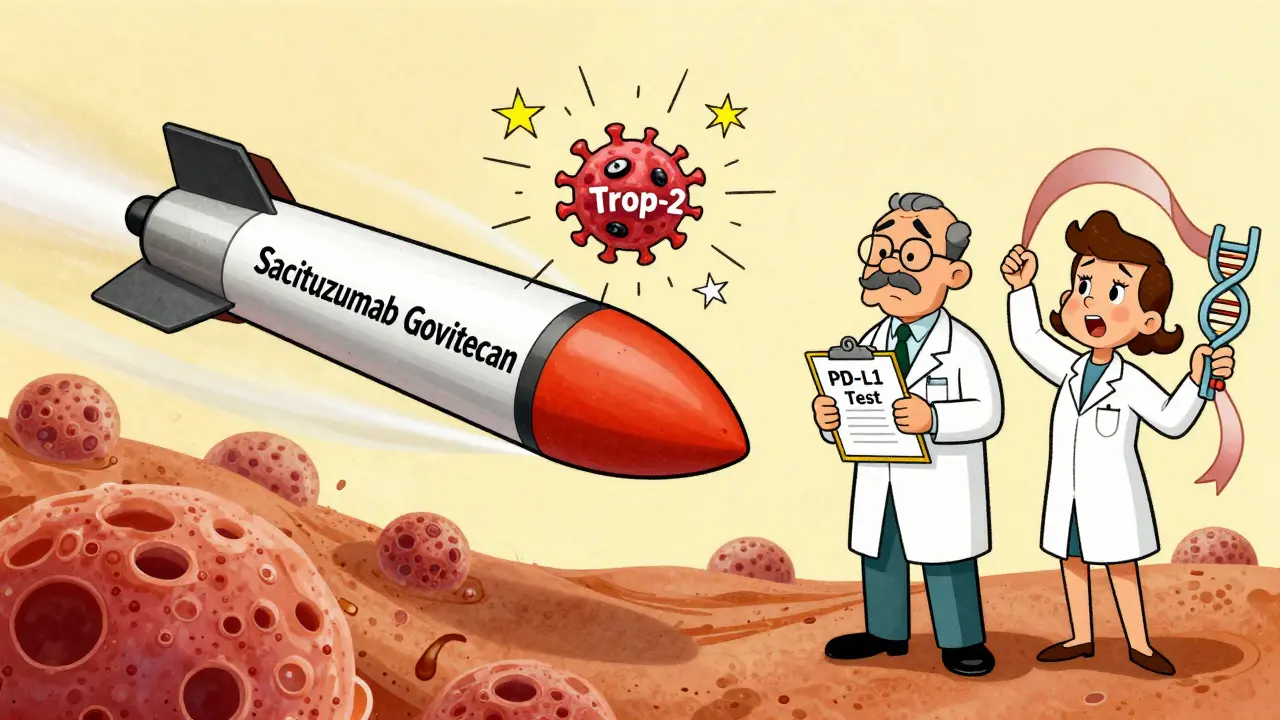
Antibody-Drug Conjugates: Smart Bombs for Cancer Cells
Sacituzumab govitecan (Trodelvy®) is one of the most effective new tools for advanced TNBC. It’s an antibody-drug conjugate-a smart missile. The antibody finds a protein (Trop-2) on TNBC cells. Once attached, it releases a powerful chemo drug directly inside the tumor.
In the ASCENT trial, it cut the risk of cancer progression by 56% and death by 57% compared to standard chemo. Response rates? 35%. Median time before cancer worsened? 5.6 months. Not perfect, but better than what came before.
Another ADC, trastuzumab deruxtecan (Enhertu®), is showing promise even in tumors with low HER2 levels-something we used to ignore. In the DESTINY-Breast04 trial, it worked in 37% of TNBC patients with minimal HER2 expression. That’s a new category of patients who might now benefit.
The Future: Personalized Vaccines and Beyond
At Houston Methodist Hospital, researchers are making custom vaccines for each TNBC patient. They take a piece of the tumor, sequence its DNA, and build a vaccine that targets the unique mutations in that person’s cancer. It’s made in-house in under six weeks.
In phase I trials, 78% of patients developed a strong immune response. When combined with pembrolizumab, the vaccine helped clear lingering cancer cells after chemo. It’s not a cure yet-but it’s the first time we’ve seen the immune system actively hunt down TNBC after treatment.
And it’s scalable. The same platform could work for pancreatic cancer, lung cancer, or any tumor with high mutation rates. If it works, this could be the first truly personalized cancer treatment that prevents recurrence-not just treats it.
What’s on the Horizon? Trials to Watch
There are over 1,500 active TNBC trials worldwide. Here are a few to keep an eye on:
- Datopotamab deruxtecan (Dato-DXd): A new TROP2-targeting ADC from Roche. Phase III results expected in late 2026.
- Adagloxad simolenin: A vaccine-like therapy that triggers immune responses against a common TNBC protein. Phase III underway.
- CDK4/6 + PI3Kα inhibitors: Dual-blockade therapy to stop cancer growth signals from two angles. Early results show tumor shrinkage in resistant cases.
- Neoadjuvant durvalumab: The GeparNuevo trial showed a 92.5% 3-year survival rate with this combo-better than chemo alone.
These aren’t just hopeful ideas. They’re moving from labs to clinics. The NCCN guidelines are expected to update in May 2026 to include several of these.
Challenges That Remain
Even with all this progress, TNBC is still tough. Five-year survival for metastatic TNBC is only 12-15%. That’s half of what it is for hormone-positive breast cancer. Resistance still happens. Tumors evolve. Some patients don’t respond at all.
Access is another problem. Biomarker testing, new drugs, and clinical trials are often only available at major cancer centers. In low- and middle-income countries, fewer than 4 in 10 patients get tested for BRCA or PD-L1. That’s not just unfair-it’s deadly.
And toxicity? Even the new drugs come with trade-offs. Sacituzumab govitecan causes severe diarrhea in 37% of patients and low white blood cell counts in 61%. Immunotherapy can trigger autoimmune reactions. We’re not done finding safer ways.
What Should You Do Now?
If you or someone you know has TNBC, here’s what to ask for:
- Full biomarker testing: BRCA (germline), PD-L1 (CPS score), HRD, tumor mutational burden.
- Discussion of neoadjuvant options: Is platinum-based chemo right? Should immunotherapy be added?
- Ask about clinical trials: Even if you’re not in a major city, many trials offer remote monitoring or travel support.
- Request a tumor board review: A team of oncologists, surgeons, radiologists, and genetic counselors should weigh in.
Don’t accept ‘standard chemo’ as the only option. Ask what’s new. Ask what’s next. Because the next step might be the one that saves your life.
Is triple-negative breast cancer curable?
Early-stage TNBC can be cured in many cases, especially with modern chemo, immunotherapy, and surgery. About 60-70% of patients with stage I-III disease achieve long-term remission. But if it spreads beyond the breast and lymph nodes (metastatic), it’s not considered curable-though it can be controlled for months or even years with new drugs like sacituzumab govitecan, PARP inhibitors, or immunotherapy combinations. Survival rates are improving, but metastatic TNBC remains the most aggressive form.
Does TNBC always come back?
No, but it’s more likely to return than other breast cancers. About 30-40% of early-stage TNBC patients see a recurrence within the first 3-5 years, mostly in the lungs, brain, or liver. After five years, the risk drops sharply. That’s why the first few years are critical. New treatments like personalized vaccines aim to reduce that risk by training the immune system to catch any leftover cancer cells before they grow.
Why is TNBC harder to treat than other breast cancers?
Because it lacks the receptors (estrogen, progesterone, HER2) that most targeted therapies are designed to block. Without those, drugs like tamoxifen or trastuzumab don’t work. That leaves chemotherapy as the main tool-which is harsh and not always effective. Plus, TNBC tumors are often genetically chaotic, making them resistant to single drugs. Now, with biomarker testing and combination therapies, we’re finally finding ways to outsmart them.
Who should get tested for BRCA mutations with TNBC?
All patients diagnosed with TNBC should get germline BRCA testing, regardless of age or family history. About 15-20% of TNBC cases are linked to inherited BRCA mutations. Finding one changes treatment: PARP inhibitors become an option, and it also informs cancer risk for family members. Testing is done through a blood or saliva sample and is now standard of care at major cancer centers.
Are there any lifestyle changes that help with TNBC treatment?
No diet or supplement has been proven to cure or directly improve TNBC outcomes. But staying physically active, eating a balanced diet, and avoiding smoking or heavy alcohol use can help your body tolerate treatment better and reduce side effects like fatigue and muscle loss. Some studies suggest maintaining a healthy weight may lower recurrence risk. Always talk to your oncology team before starting any new supplement-some can interfere with chemo or immunotherapy.
How do I find a clinical trial for TNBC?
Start with your oncologist-they often know about local or national trials. You can also search ClinicalTrials.gov, which lists all active studies in the U.S. and many worldwide. Filter by ‘triple-negative breast cancer,’ your location, and treatment phase (I, II, or III). Many trials cover travel, lodging, or drug costs. Don’t assume you’re not eligible-many trials now accept patients who’ve had prior treatment, and some are designed specifically for those who’ve relapsed.
What Comes Next?
The next five years will define how we treat TNBC. We’re moving from one-size-fits-all chemo to treatments chosen by your tumor’s DNA, your immune profile, and even your personal mutation fingerprint. Vaccines, dual-target drugs, smarter ADCs-they’re not science fiction anymore. They’re in clinics.
The biggest win won’t be a single miracle drug. It’ll be the system: test early, test often, match precisely, and treat smartly. That’s the new standard. And for the first time, it’s giving people with TNBC a real shot at long-term survival.




4 Comments
Chemo before radiation? That’s backwards. Everyone knows you hit hard first then clean up. This new protocol is just corporate fluff to cut costs. I’ve seen patients crash harder with this combo. Trust me I’ve been through it twice.
They say PARP inhibitors work for BRCA but what if the test is wrong? What if the lab messed up? What if Big Pharma is hiding that 30% of these drugs only work because patients are dying faster and they count it as ‘progress’? I’m not buying it.
This gave me hope 🥹 I lost my mom to TNBC in 2020 and reading about these vaccines and ADCs… I actually cried. Not because it’s perfect but because we’re finally moving. Thank you for writing this.
As someone from a rural community where testing isn’t even offered, I’m so glad this is being shared. My sister got diagnosed last year and they didn’t even mention BRCA until she found this article herself. Please keep pushing awareness. 🌍❤️
Write a comment