Graves’ disease is the most common cause of hyperthyroidism in places where people get enough iodine in their diet. It happens when your immune system accidentally attacks your thyroid gland, telling it to make way too much hormone. This isn’t just a slow burn-it can hit hard and fast, changing how you feel, sleep, think, and even look. About 1 in 50 women and 1 in 200 men will develop it in their lifetime, with most cases showing up between ages 30 and 50. If you’ve been feeling anxious, shaky, or like you’re always overheating despite the AC running, this might be why.
What Happens Inside the Body?
Your thyroid is a butterfly-shaped gland in your neck that controls your metabolism. In Graves’ disease, your body makes strange antibodies called thyroid-stimulating immunoglobulins (TSI). These act like fake keys that fit perfectly into the thyroid’s TSH receptors. Instead of turning off, your thyroid goes into overdrive, pumping out excess T3 and T4 hormones. The result? Your whole system speeds up.
Doctors call this autoimmune hyperthyroidism because the immune system is the root cause. Unlike other thyroid problems-like a nodule or inflammation-Graves’ is systemic. That means it doesn’t just affect the thyroid. It can show up in your eyes, your skin, your heart, and even your bones. About 30% of people with Graves’ develop eye symptoms. In severe cases, the eyeballs bulge out (exophthalmos), vision blurs, and double vision kicks in. This isn’t cosmetic-it’s functional. Some patients lose the ability to read or drive safely until treated.
How Do You Know You Have It?
The symptoms are broad but recognizable. Younger people often feel like they’re wired: racing heart, trouble sleeping, weight loss even with a huge appetite, trembling hands, and sweaty palms. Older adults might not show the classic signs. Instead, they get heart palpitations, chest discomfort, memory lapses, or just feel weak all the time. A simple physical exam can raise red flags-like a fast pulse (over 100 bpm), an enlarged thyroid, or eye changes.
But symptoms alone aren’t enough. Diagnosis needs lab tests. The first step is checking your TSH level. In Graves’, it’s almost always below 0.4 mIU/L because your brain says, “Stop making hormone!” Then, your free T4 and T3 levels are measured. If T4 is above 1.8 ng/dL and T3 above 180 ng/dL, that’s a strong signal. But the real clincher? Antibody testing. Over 90% of people with Graves’ test positive for TSH receptor antibodies (TRAb) or TSI. No other condition mimics this pattern exactly. If antibodies are positive, you don’t need a radioactive scan-this is Graves’.
For cases where antibody tests aren’t available, doctors may check thyroid blood flow using ultrasound. Studies show this method is over 90% accurate in spotting Graves’ by detecting the increased flow that comes with overactive tissue.
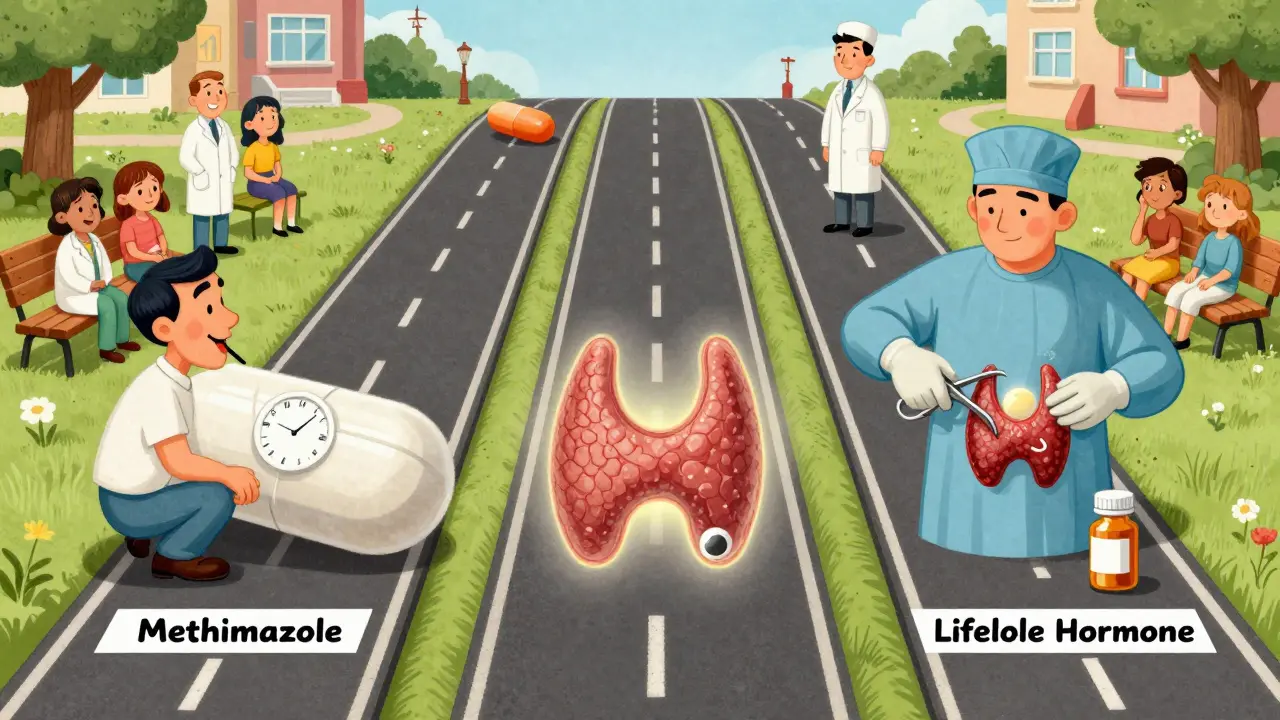
Three Main Treatment Paths
There are three standard ways to treat Graves’ disease-and no single one is right for everyone. The choice depends on your age, how bad your symptoms are, whether you have eye problems, and what you’re willing to live with long-term.
1. Antithyroid Medications
Most people start here. Methimazole is the go-to drug. It blocks your thyroid from making extra hormone. It’s taken once a day, usually 10 to 40 mg. Propylthiouracil (PTU) is an alternative, but it’s riskier-about 1 in 500 people on PTU develop liver damage. Methimazole’s liver risk is lower, around 1 in 1,000.
Treatment lasts 12 to 18 months. During that time, you’ll need monthly blood tests to check your hormone levels and white blood cell count. Why? Because methimazole can rarely cause agranulocytosis-a dangerous drop in infection-fighting cells. If you get a fever or sore throat, stop the pill and get checked immediately.
Success? About 30% to 50% of people go into remission after treatment ends. That means their thyroid returns to normal without drugs. Remission is more likely if your thyroid is small, your antibodies drop to zero before stopping meds, and you don’t smoke. But if the disease comes back? You’re back to square one.
2. Radioactive Iodine (I-131)
This is the most common long-term solution in the U.S. You swallow a pill containing a small dose of radioactive iodine. Your thyroid absorbs it like normal iodine, and the radiation gently destroys the overactive cells. It’s not painful. You don’t feel anything.
Within 6 to 12 months, most people become hypothyroid. That sounds scary, but it’s actually predictable. You’ll start taking levothyroxine daily-usually 1.6 mcg per kg of body weight. It’s a simple pill, taken on an empty stomach. Once adjusted, you’ll feel normal again.
The big advantage? One-time treatment. No daily pills after the first year. The downside? You’ll be on thyroid hormone for life. Some people regret this if they weren’t fully warned. Studies show 55% of patients who chose radioactive iodine wish they’d known more about lifelong replacement before deciding.
3. Thyroid Surgery (Thyroidectomy)
Surgery removes the thyroid entirely. It’s not first-line unless you have a huge goiter pressing on your windpipe, can’t take meds due to allergies, or have severe eye disease that’s getting worse. It’s also chosen by people who want to avoid radiation or can’t wait months for radioactive iodine to work.
The success rate is over 95%. But it’s not risk-free. There’s a 1-2% chance of damaging the parathyroid glands, which control calcium. A 0.5-1% risk of injuring the voice box nerve can lead to hoarseness. Recovery takes a few weeks. After surgery, you’ll need lifelong thyroid hormone replacement, just like with radioactive iodine.
What About the Eyes?
Graves’ eye disease-also called thyroid eye disease or Graves’ ophthalmopathy-is separate from the thyroid overactivity. You can have eye symptoms without high hormone levels, and vice versa. Smoking makes it 7 to 8 times worse. Quitting is the single most effective thing you can do to stop progression.
For mild cases, selenium supplements (100 mcg twice daily for 6 months) help reduce swelling and discomfort. For moderate to severe cases, doctors use high-dose IV steroids-500 mg of methylprednisolone weekly for 6 weeks, then 250 mg weekly for another 6 weeks. About 60-70% of patients improve.
There’s a newer option: teprotumumab. It’s a monoclonal antibody that targets the insulin-like growth factor receptor, which drives eye inflammation. In clinical trials, 75-80% of patients saw their eyeballs retreat by at least 2 mm. About 69% had clinically meaningful improvement. It’s not cheap, but for those with vision-threatening eye bulging, it’s life-changing.

Life After Diagnosis
Getting diagnosed is just the start. Managing Graves’ means learning your body’s new rhythm. You’ll need regular blood tests-monthly at first, then every few months. Missing doses of methimazole increases relapse risk by 40-50%. Sleep, stress, and diet matter too. Many patients report feeling 80% better within 3 months of getting hormone levels stabilized.
One patient on a support forum said, “My heart palpitations vanished in two weeks after my methimazole dose hit 15 mg.” Another, after orbital decompression surgery: “I could read again. I could drive. I didn’t feel like I was staring out of a fishbowl.”
But it’s not all smooth sailing. Some struggle with fatigue for months. Others deal with weight gain after treatment, not because of the disease, but because their metabolism finally slows down. Depression and anxiety can linger even after hormone levels normalize. Support groups help. So does patience.
What’s on the Horizon?
Research is moving fast. Scientists are testing drugs that target specific immune cells involved in Graves’. Rituximab, which wipes out B-cells, showed 60% response rates in early trials for severe eye disease. Genetic studies have found 12 gene variants linked to higher risk-like HLA-DQA1 and CTLA4. Understanding these could lead to personalized prevention someday.
For now, the best advice remains: get tested early, treat aggressively, quit smoking, and don’t ignore the eyes. Graves’ disease isn’t a death sentence. It’s a manageable condition-with the right tools, you can live fully again.
Can Graves’ disease be cured without medication?
In some cases, yes-but not reliably. About 30-50% of people who take antithyroid drugs for 12-18 months go into remission and never need medication again. However, if antibodies remain high or the thyroid is large, relapse is likely. There’s no guaranteed cure without treatment. Radioactive iodine and surgery are considered permanent solutions because they remove or destroy the overactive thyroid tissue. But even then, you’ll need lifelong hormone replacement.
Is Graves’ disease hereditary?
Yes, it runs in families. About 25-30% of people with Graves’ have a close relative with an autoimmune thyroid disorder. You don’t inherit the disease directly, but you inherit genes that make your immune system more likely to attack the thyroid. Genes like HLA-DQA1 and CTLA4 are strongly linked. If a parent or sibling has it, your risk is higher-but still low overall. Most people with these genes never develop Graves’.
Why do women get Graves’ disease more often than men?
Women are 7 to 8 times more likely to develop Graves’ disease. The exact reason isn’t fully known, but hormones play a big role. Estrogen affects immune system activity, and women experience more immune fluctuations during pregnancy, postpartum, and menopause. In fact, the risk spikes 3 to 5 times in the year after giving birth. Autoimmune conditions in general are more common in women, likely due to this complex interaction between sex hormones and immune regulation.
Can stress cause Graves’ disease?
Stress doesn’t cause Graves’ disease, but it can trigger it in people who are genetically at risk. Major life events-like the death of a loved one, a divorce, or serious illness-can activate the immune system in a way that leads to autoimmunity. Studies show that up to 40% of newly diagnosed patients report a major stressor within 6 months before symptoms began. It’s not the cause, but it’s often the spark.
Will I gain weight after treatment?
Many people do-especially after radioactive iodine or surgery. While Graves’ disease speeds up your metabolism and causes weight loss, treatment slows it back down. If you don’t adjust your diet or activity level, weight gain is common. It’s not because you’re eating too much-it’s because your body is returning to normal. Most patients gain 5 to 15 pounds after treatment. Working with a dietitian and staying active can help manage this.