Tag: generic equivalence

Bioequivalence Explained: FDA Requirements to Prove Generic Drug Equivalence
- By : Tamsin Riverton
- Date : Feb 17 2026
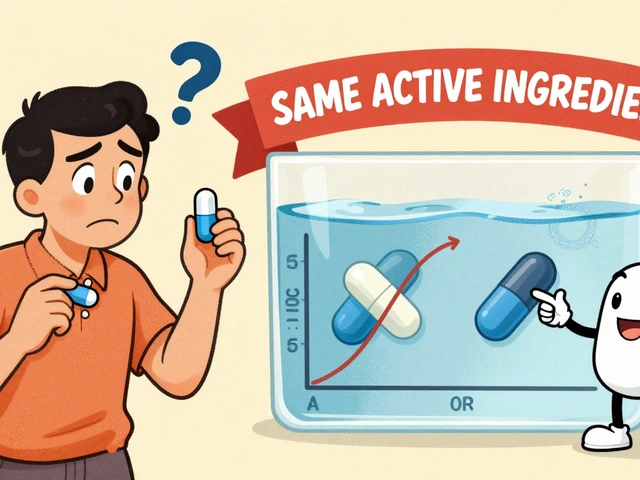
Bioequivalence is the scientific standard the FDA uses to ensure generic drugs work the same as brand-name versions. It's not about price - it's about how your body absorbs the medicine. Here's how it works.
Popular Posts
Tags
side effects
online pharmacy
medication safety
generic drugs
drug side effects
brand vs generic
medication adherence
generic drug approval
ANDA approval
blood pressure
dosage
online pharmacy UK
herbal adaptogen
eGFR
ED medication comparison
treatment options
antibiotic comparison
bleeding risk
prior authorization
antihistamines