Tag: generic medication
What Is an ANDA? The Complete Guide to Abbreviated New Drug Applications and FDA Approval
- By : Tamsin Riverton
- Date : Dec 29 2025
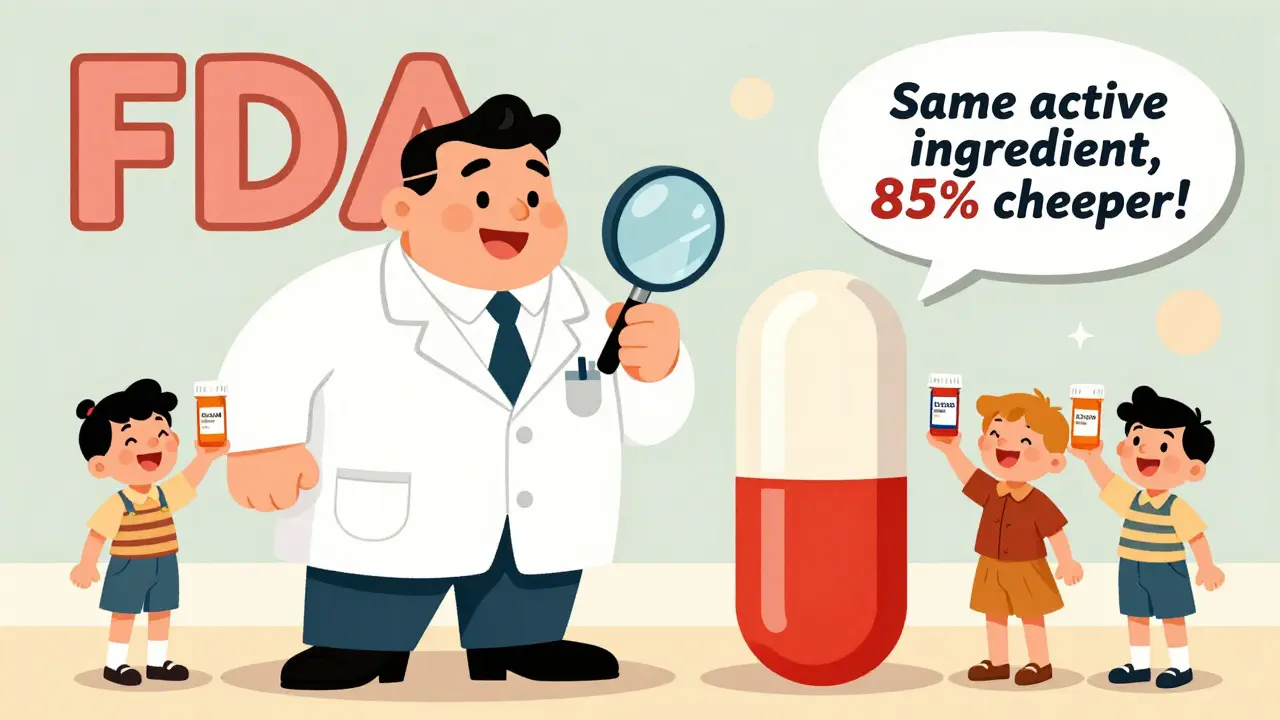
An ANDA, or Abbreviated New Drug Application, is the FDA pathway that allows generic drugs to be approved without repeating expensive clinical trials. It ensures safe, effective, and affordable medications for millions.
Popular Posts
Tags
side effects
online pharmacy
medication safety
generic drugs
drug side effects
brand vs generic
medication adherence
generic drug approval
blood pressure
dosage
online pharmacy UK
herbal adaptogen
eGFR
ED medication comparison
treatment options
antibiotic comparison
bleeding risk
prior authorization
antihistamines
FDA generics