Category: Health - Page 3

FDA Generic Drug Approval: Step-by-Step Process for ANDA Submission
- By : Tamsin Riverton
- Date : Dec 31 2025
Learn the complete step-by-step FDA generic drug approval process through the ANDA pathway, including pharmaceutical equivalence, bioequivalence testing, CMC requirements, and how generics save billions in healthcare costs.

Australia's Generic Market: PBS Overview and Impact
- By : Tamsin Riverton
- Date : Dec 30 2025
Australia's Pharmaceutical Benefits Scheme (PBS) subsidizes prescription drugs, making generics affordable for millions. Learn how the system works, its impact on drug prices, and why 84% of prescriptions are generic.
What Is an ANDA? The Complete Guide to Abbreviated New Drug Applications and FDA Approval
- By : Tamsin Riverton
- Date : Dec 29 2025
An ANDA, or Abbreviated New Drug Application, is the FDA pathway that allows generic drugs to be approved without repeating expensive clinical trials. It ensures safe, effective, and affordable medications for millions.
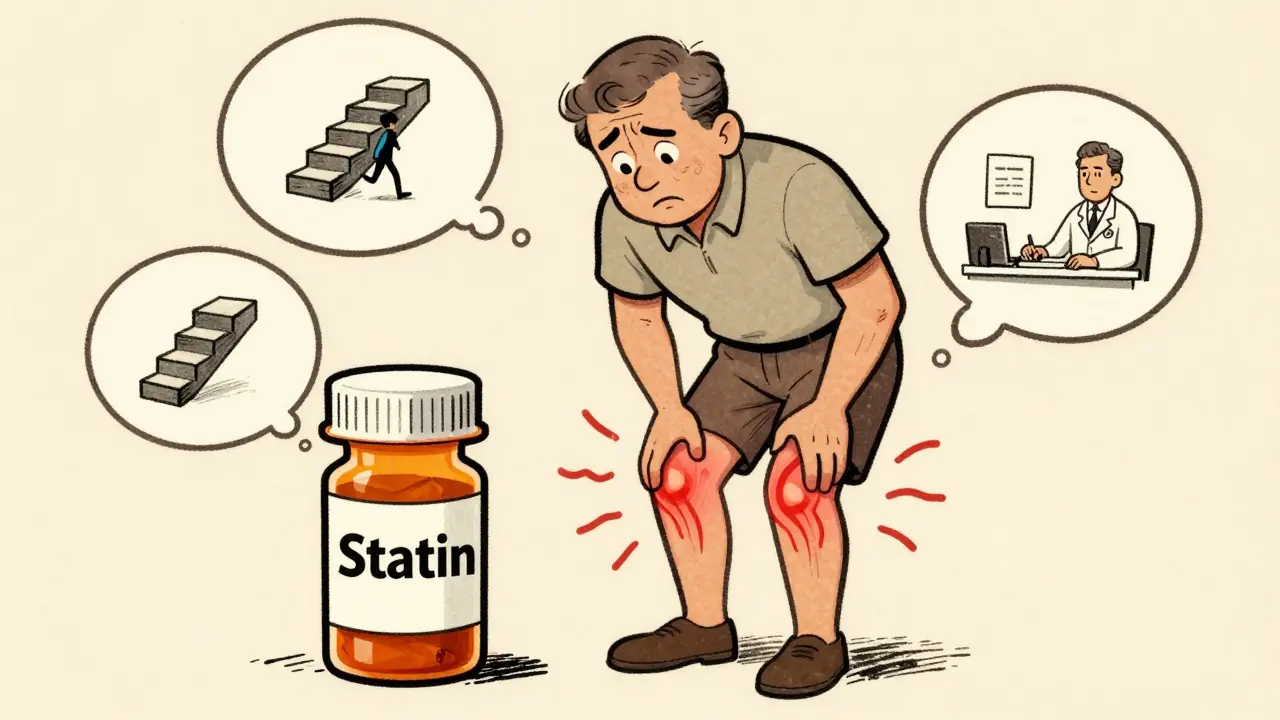
Muscle Aches from Statins: What to Do When Medication Causes Pain
- By : Tamsin Riverton
- Date : Dec 28 2025
Muscle aches from statins are common but often misunderstood. Learn what causes them, when to worry, and how to manage them without risking your heart health.
Kava and Liver Health: Safety with Other Medications
- By : Tamsin Riverton
- Date : Dec 27 2025
Kava may help with anxiety, but it can cause serious liver damage - especially when mixed with common medications. Learn which drugs are dangerous with kava, who's at risk, and what to do if you're already using it.

AKI on CKD: How to Avoid Contrast and Nephrotoxic Medications
- By : Tamsin Riverton
- Date : Dec 25 2025
AKI on CKD is a life-threatening complication. Avoid contrast dye and nephrotoxic medications like NSAIDs to protect your kidneys. Learn the real risks and what to do now.

The Purple Book: Understanding Biosimilars and Interchangeability from the FDA
- By : Tamsin Riverton
- Date : Dec 24 2025
The FDA's Purple Book is the official database for biosimilars and interchangeable biological products. Learn how it works, which products are substitutable, and why state laws still control pharmacy substitutions.

Cancer Pain Management: Opioids, Nerve Blocks, and Integrative Care
- By : Tamsin Riverton
- Date : Dec 23 2025
Cancer pain affects nearly half of all patients. Learn how opioids, nerve blocks, and integrative therapies like acupuncture and mindfulness work together to provide real relief-backed by the latest research and clinical guidelines.
Manufacturing Challenges for Biosimilars: Complex Production and Why It’s Not Like Generic Drugs
- By : Tamsin Riverton
- Date : Dec 22 2025
Biosimilars aren't simple copies like generics-they're complex biologics made in living cells. This article breaks down the real manufacturing challenges: glycosylation, scale-up, cold chain risks, regulatory hurdles, and how new tech is helping manufacturers keep up.

Tentative Approval for Generics: Common Reasons for Delays
- By : Tamsin Riverton
- Date : Dec 21 2025
Tentative approval from the FDA means a generic drug is scientifically ready - but not yet legal to sell. Common delays include patent lawsuits, manufacturing issues, and market economics that prevent launch even after approval.

Generational Differences in Attitudes Toward Generic Medications
- By : Tamsin Riverton
- Date : Dec 20 2025
Generational attitudes toward generic medications vary widely, not because of age alone, but due to health literacy, brand exposure, and trust in medical systems. Understanding these differences helps bridge the gap between science and perception.
Why Brand Companies Launch Authorized Generics: Strategy Explained
- By : Tamsin Riverton
- Date : Dec 19 2025
Brand companies launch authorized generics to protect revenue, block generic monopolies, and keep customer trust. These are the exact same drugs, sold cheaper - and they're reshaping how pharmaceutical markets work.